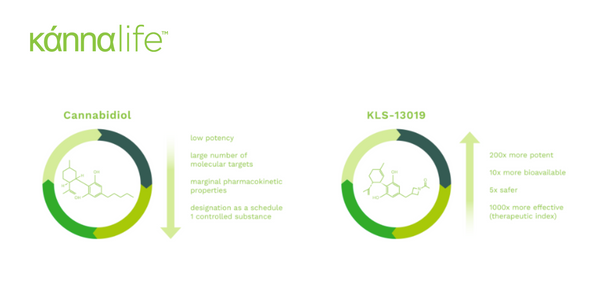
DOYLESTOWN, Penn., Jan. 14, 2020 -- Kannalife, Inc. (“Kannalife” or the “Company”) (OTCQB: KLFE), a biopharmaceutical company specializing in the research and development of cannabinoid (CBD) therapeutics, announced today that it has further elucidated the mechanism of action (MOA) of its leading investigative drug candidate KLS-13019, a CBD-like molecule for the potential treatment of neuropathic pain. Through extensive pre-clinical testing and under a NIH-NIDA Phase 1 grant study, Kannalife has identified a potent, non-opioid alternative to the frontline treatment of chemotherapy-induced peripheral neuropathy (CIPN). Under the recently completed NIH-NIDA Phase 1 grant study, Kannalife’s research has shown that its leading drug candidate KLS-13019 plays an important role in the regulation of mNCX-1, an important pharmacological target in the prevention and in-part reversal of CIPN in the pre-clinical animal model. In comparison with CBD and morphine, KLS-13019 stood out as a viable drug candidate that out-performed CBD and poses as a viable alternative to the use of opioids as the frontline current standard of care for patients suffering from CIPN.
Chemotherapy-induced peripheral neuropathy is a disabling pain condition that afflicts between 30-40 percent of patients undergoing chemotherapy, for which there is no effective prevention strategy and treatment of established chronic CIPN is limited.1 Existing treatment options primarily include anticonvulsants and antidepressants, as well as opioids in more severe cases of CIPN.2
“There is a tremendous unmet patient need when it comes to treating neuropathic pain disorders, particularly CIPN. We believe we are on the forefront of a breakthrough in non-opioid alternatives for the treatment of chronic pain management, for which KLS-13019 represents a potential new non-addictive option with greater selectively and a wider therapeutic window of use. The target mNCX-1 is just part of the big story here. Stay tuned,” stated Dean Petkanas, CEO of Kannalife.
The global market for neuropathic pain was valued at more than $5 Billion in 2015, and in 2016, CIPN accounted for more than 42% of market revenue. It’s estimated that by 2024, the total global neuropathic pain market will be worth more than $8.3 Billion.3-4
To further bring to life the MOA of KLS-13019 as well as Kannalife’s data-driven, scientific approach to the discovery of CBD-like molecules, the Company has launched a new animated video via Vimeo at https://vimeo.com/371214213. Through its research and development efforts over the past decade, the Company has established an intellectual property estate specific to the formulation of these CBD-like compounds.
Additional scientific details of the MOA as well as the protective effects of CBD and KLS-13019 against CIPN toxicity have been published in the Journal of Molecular Neuroscience in a paper titled, “Knockdown siRNA Targeting the Mitochondrial Sodium-Calcium Exchanger-1 Inhibits the Protective Effects of Two Cannabinoids Against Acute Paclitaxel Toxicity.”
About KLS-13019
KLS-13019 is Kannalife’s leading proprietary investigational CBD-like product for the potential treatment of a range of neurodegenerative and neuropathic pain disorders, beginning with chemotherapy-induced peripheral neuropathy (CIPN). KLS-13019 has not been reviewed or approved for patient use by the U.S. Food and Drug Administration (FDA) or any other healthcare authority in the world. It’s safety and efficacy have not been confirmed by FDA-approved research.
About Kannalife, Inc.
Kannalife, Inc. is a biopharmaceutical company focused on the development of proprietary and patented cannabidiol (CBD) and CBD-like molecules for patients suffering from unmet medical needs of neurodegenerative disorders - including chemotherapy-induced peripheral neuropathy (CIPN), a chronic neuropathy caused by toxic chemotherapeutic agents; hepatic encephalopathy (HE), a neurotoxic brain-liver disorder caused by excessive concentrations of ammonia and ethanol in the brain; mild traumatic brain injury (mTBI), a disorder associated with single and repetitive impact injuries; and chronic traumatic encephalopathy (CTE) a disease associated with highly repetitive impact injuries in professional and amateur sports.
The Company's family of proprietary molecules focuses on treating oxidative stress-related diseases such as HE, chronic pain from neuropathies like CIPN, and neurodegenerative diseases like CTE. Kannalife conducts its research and development efforts at the Pennsylvania Biotechnology Center of Bucks County in Doylestown, PA.
For more information about Kannalife, Inc., visit www.kannalife.com and visit the Company’s Twitter page at @Kannalife.
Forward-Looking Statements
This press release may contain certain forward-looking statements and information, as defined within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934 and is subject to the Safe Harbor created by those sections. This press release contains statements about expected future events, the company’s business plan, plan of operations, the viability of the company’s drug candidates, and/or financial results that are forward-looking in nature and subject to risks and uncertainties. Such forward-looking statements, by definition, involve risks and uncertainties. The company does not sell or distribute any products that are in violation of the United States Controlled Substances Act.
References
- National Center for Biotechnology Information. Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: a systematic review and meta-analysis. Accessed at https://www.ncbi.nlm.nih.gov/books/NBK291675/.
- Datamonitor Healthcare. Neuropathic Pain Market and Forecast Analysis to 2026. Accessed at https://pharmastore.informa.com/product/neuropathic-pain-market-forecast-analysis-2026/.
- BioSpace. Neuropathic Pain Market: lucrative opportunities and Fast Growth by 2026. Accessed at https://www.biospace.com/article/neuropathic-pain-market-lucrative-opportunities-and-fast-growth-by-2026/.
- Research Reporting Insights. Neuropathic Pain Market. Accessed at https://www.researchreportinsights.com/report/sample/110114956/Neuropathic-Pain-Market.